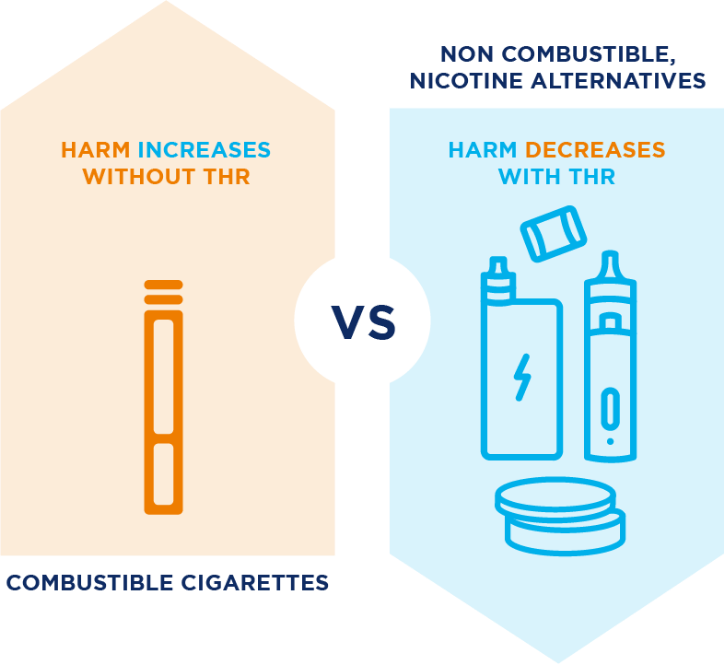
New Research Supports the Role of Smokeless Product Alternatives in Tobacco Harm Reduction
At the Reynolds American organization, we believe science should be more than a set of data points—it should also lead to meaningful change. That’s why our commitment to Tobacco Harm Reduction (THR) is grounded in science, transparency, and real-world evidence. Through THR, we aim to create A Better Tomorrow™ by Building a Smokeless World—one … Continued