Global Tobacco & Nicotine Forum
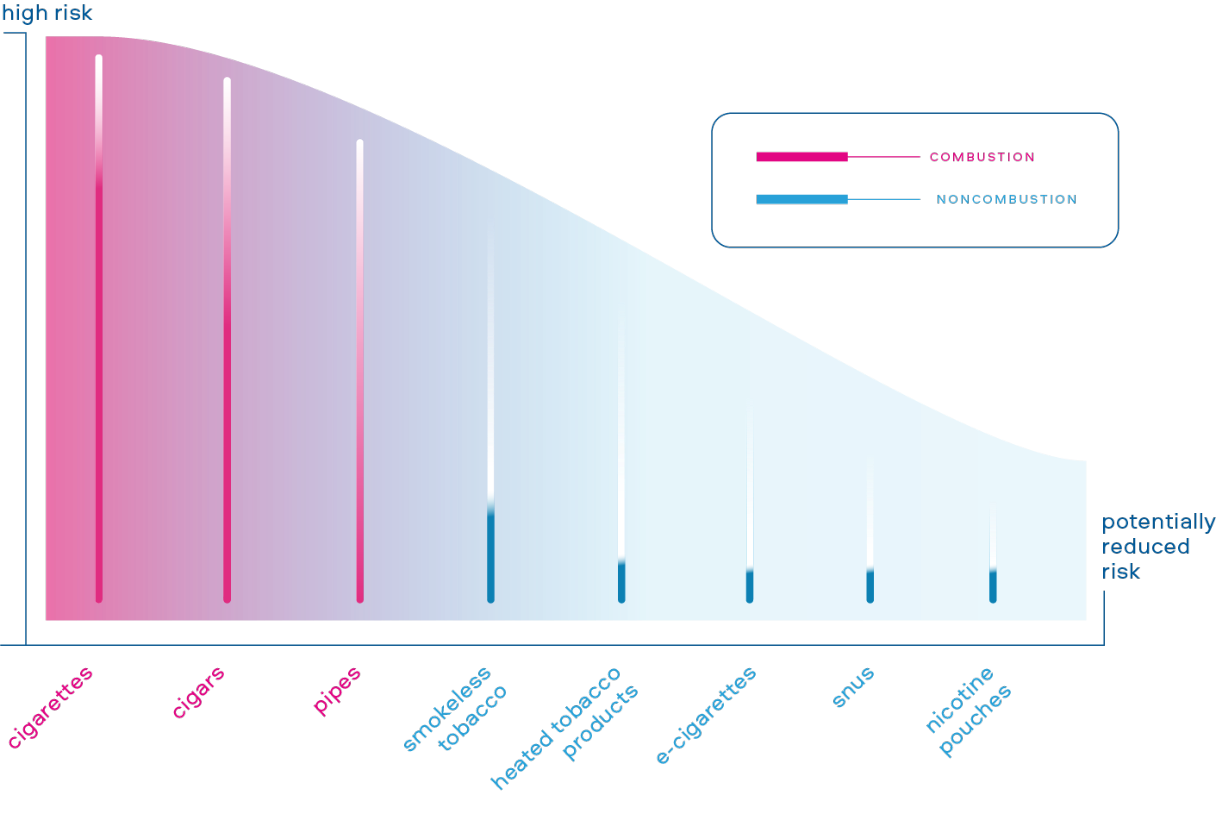
The annual Global Tobacco & Nicotine Forum in Washington, DC, has been a global exchange of views since it was founded in 2008. It attracts high-profile scientists, public health experts, government officials and investors. In 2022, our colleagues participated in several panel events, speeches and engagements focused on Tobacco Harm Reduction.